had the pleasure of attending the IMCAS Annual World Congress in Paris last January as part of the Brera Medical Technologies global experts of Plasmage® (I am aesthetics physicians based in Naples, Italy).
With over 200 exhibiting companies and more than 7,000 dermatologists, plastic surgeons, and other aesthetic practitioners from 85 countries, IMCAS is one of the largest multi-specialty conferences dedicated to aesthetic medicine.
More than 300 doctors visited the Brera stand in 3 days, which, as any experienced marketer will know, is a lot of attention! Why so many visits? Well, most were to find out more about Plasmage®, a Fractional Plasma ® energy device poised to revolutionise reconstructive eyelid treatments.
During the congress, Brera also organised a consensus meeting with experienced practitioners already using Plasmage®. The aims of this meeting were to get our feedbacks on the device and to kick off the largest clinical study ever organised for plasma energy-based eyelid treatments, led by Dr Welf Prager (Hamburg, Germany).
Other aesthetic practitioners present at the consensus meeting included Dr Shaimaa Embaby (Cairo, Egypt), Dr Devrim Gürsoy (Ankara, Turkey), Dr Serkan Öztuk (Ankara, Turkey), Dr Christine Diericks (Boom, Belgium), Dr Muhannad Adad (Amman, Jordan), Dr Medhat Malek (Amman, Jordan), Dr Pedro Melo (Lisbon, Portugal), Dr Jacques André David (Paris, France), Dr Pepe José Contreras (Madrid, Spain), who had each treated between 20 and 100 patients with the Plasmage® system.
During the meeting, I learnt:
1) Fractional plasma ® is a revolutionary approach to reconstructive and cosmetic eyelid treatment
Plasma energy technology emerged as a non-invasive dermatologic treatment in 2007 but made a slow entry into the market due to its hard-to-control, continuous energy delivery, which can put patients at risk for injury. Only a handful of experienced practitioners used the technology, obtaining mixed results.
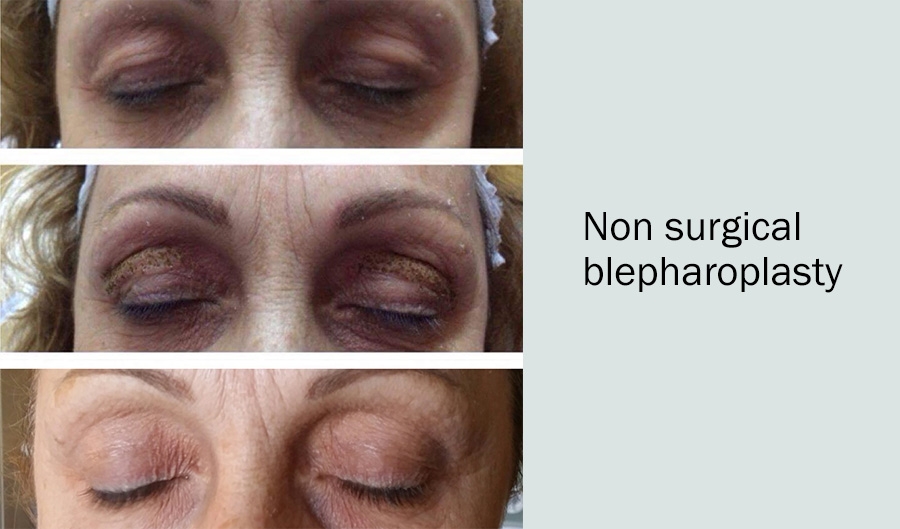
Through the Plasmage® system, Brera Medical Technologies has made fractional plasma ® energy delivery possible. Fractional plasma ® delivery provides the same power capacities, yet is safer to the skin and more easily controlled. Ultimately, skin recovery time is shorter and, according to the 20 dermatologists I spoke with, the end results are stunning. Moreover, for patients seeking reconstructive eyelid treatments, Plasmage® represents the only non-invasive alternative to blepharoplasty surgery.
2) Eyelid procedures take 15 minutes and end results take just 6−8 weeks
Skin recovery time is always a concern to patients, even if they can conceal treated eyelids with sunglasses as they heal.
Patients can expect the following from Plasmage® eyelid treatments:
1. Anaesthetic cream (10% lidocaine) is applied to treatment areas for 30−40 minutes.
2. Both upper eyelids are treated; the operation lasts around 15 minutes.
3. A cortisone cream is applied to treated skin regions for 3 days, along with sunblock cream and sunglasses. No other medication is needed.
4. Crusts on the skin fall away 5−7 days after treatment. The patient must continue to wear sunblock and sunglasses.
5. After 6−8 weeks, the skin is fully renewed.
3) The preset parameters of Plasmage ® work well
All the physicians (aesthetic doctors, dermatologist and surgeons) present at the meeting were using the preset power and frequency parameters of the Plasmage® system during treatments. They explained that it appears to be the right compromise between efficacy and safety.
Several of the experts explained that they were finishing treatments with a few additional touch-ups at a lower power.
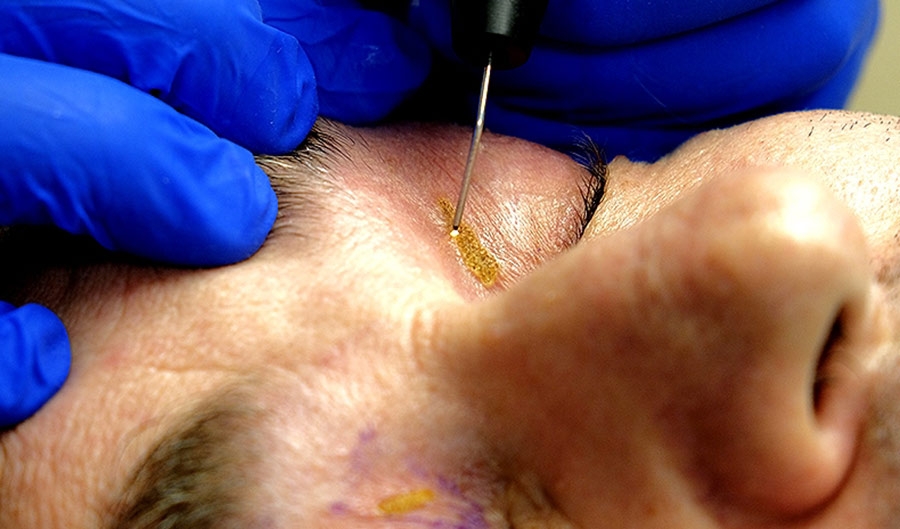
4) Plasma energy is best administered in a 1 mm-spaced dotted pattern over a large area
Two intraoperative techniques were discussed for eyelid treatments.
The first consists of treating only the skin to be removed, accessible by gently pressing on the eyebrow to create a fold on the eyelid. Treatment is performed by delivering successive plasma doses in a dotted formation, separated by about 1 mm of intact tissue, to allow recovery without hyperpigmentation. The results shared by practitioners using this technique showed excellent efficacy.
However, the best results were seen from the second technique, which involves treating a larger surface of the eyelid from the eyebrow to the eyelash line. This technique also involved plasma doses separated by 1 mm of intact tissue.
5) Clinical study publications are on their way
Consensus protocols will be published during summer, 2017. A meta-analysis should be released by June 2017 along with the first phase of a large multi-centre study. These three articles will focus on reconstructive eyelid treatments for Fitzpatrick skin phototypes I to IV.
A phase II study is also on its way, which will compare Fractional Plasma ® with other cosmetic interventions in skin phototypes I to IV.
by Dr Anna Maria d’Ardis
Additional Info
-
Item Shortcode:

5 things I learnt during the Plasmage®
Domenica, 04 Novembre 2018 | Written by Dr Anna Maria d’Ardis |Consensus Meeting at the 2017 IMCAS World Congress, Paris